Monday, April 27, 2009
Streaks and dark bands

Scalloping Artefacts

Artefacts


Tuesday, April 21, 2009
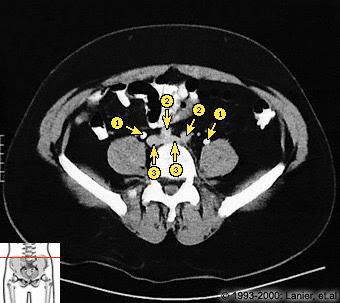
SEGMENTATION

Segmentation can be performed manually or
(semi)automatically. Segmentation algorithms are
often based on the principle of region growing
. Placing one or more seed points initiates the
segmentation of the target structure. From these
seed points, more and more neighboring voxels
that fulfill predefined criteria are included in the
segmentation . The technique can be applied
in two ways: segmentation of the desired tissue or
segmentation of the undesired tissue with subsequent
removal from the data. The latter method
removes only interfering tissue (bone or densely
enhanced veins) from the CT angiography data
and retains soft tissue as well as contrast-enhanced
vessels for further evaluation. To refine
the boundary of the segmented structures, morphologic
dilation operations may be applied.
A particular problem in threshold-based segmentation
algorithms are areas with close contact
of two tissue types with comparable attenuation,
such as bone and contrast-enhanced vessels
(course of the ICA through the skull base; intraforaminal
sections of the vertebral artery)
. Although the process of segmentation is
semiautomatic, user interaction is necessary to set
additional seeding points or to intervene in cases
of inclusion of neighboring structures due to leakage
of the region-growing algorithm. These procedures
can be time-consuming and may exceed
practical limits in routine clinical work flow.
MIP


MIP images are created by displaying only the
highest attenuation value from the data encountered
by a ray cast through an object to the viewer’s
eye (5,6). The depth information along the
projection ray is lost; to visualize the spatial relationship
of various structures, the volume has to
be rotated and viewed from different angles. If
bone or calcifications are within the projection
ray, these structures are represented on the MIP
image instead of the contrast-enhanced vessel
because of higher attenuation values. Therefore,
bone elimination techniques are essential for pro-
cessing vascular MIP images. Superimposition of
vessels leads to artificially altered lumen margins,
and pathologic conditions may be hidden. To
cope with this problem, a modification of MIP
called closest vessel projection has been proposed
(7). Thin-slab MIP images viewed interactively
may be an alternative, as the necessity for bone
elimination is limited. MIP is not suitable
for the evaluation of stenosis in cases of dense
calcification or stents, but thin-slab MIP can provide
an excellent “road map” of the vessel course
for further evaluation with MPR.
Bolus tracking
Individual timing of contrast material
injection (bolus tracking or test bolus injection)
is mandatory to take advantage of phaseresolved
image acquisition.
To individualize the timing of contrast material
injection, automatic bolus tracking techniques
(Smart Prep, CARE Bolus, and Sure Start) can
be employed (2). These techniques are fast and
easy to use and require only a single contrast material
injection. The disadvantage is that a large
target vessel for monitoring the contrast material
arrival is required, and an additional delay for
table movement and patient instruction is necessary.
Test bolus injection is the alternative to assess
the individual circulation time. Its major advantage
is that it provides information about the timing
of both arterial and venous enhancement in
the vessels of interest. The individual start
delay can be optimized by placing the scan between
the arterial peak and venous contrast material
upslope. Table movement and patient instructions
can be performed prior to the optimal
image acquisition window. The disadvantage is
the necessity for an additional injection of about
10 mL of contrast agent (10%–20% increase of
total amount).
Wednesday, March 11, 2009
Tuesday, March 10, 2009
hepatic circulation
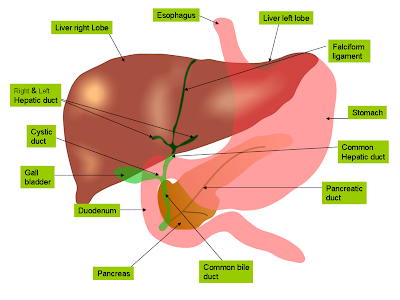
The liver receives its oxygen from a small hepatic artery that branches off the coelic artery, but most of the blood flowing through the liver comes from the gut. All the blood delivered by the coeliac artery, the anterior mesenteric artery and the posterior mesenteric artery is collected into the large hepatic portal, which runs parallel to the common bile duct. Usually, the hepatic portal does not get injected with latex, so it can be difficult to see.
After it enters the liver, the hepatic portal distributes blood to a vast network of sinuses in the liver, where it can be screened and its solutes adjusted. These sinuses are then drained by small veins which merge to form the hepatic vein. The hepatic vein, shown in the photo, was buried right in the tissue of the liver, and had to be dissected out. The hepatic vein then takes blood to the posterior vena cava.
Monday, March 9, 2009



Celiac.
Superior mesenteric
Middle suprarenaL
Lumbar
Inferior mesenteric
Median sacral
Common iliac
They may arise separately from the front of the aorta, immediately above the celiac artery, or by a common trunk, which may spring either from the aorta or from the celiac artery. Sometimes one is derived from the aorta, and the other from one of the renal arteries; they rarely arise as separate vessels from the aorta.
They diverge from one another across the crura of the diaphragm, and then run obliquely upward and lateralward upon its under surface.
The left phrenic passes behind the esophagus, and runs forward on the left side of the esophageal hiatus.
The right phrenic passes behind the inferior vena cava, and along the right side of the foramen which transmits that vein.
Near the back part of the central tendon each vessel divides into a medial and a lateral branch.
The medial branch curves forward, and anastomoses with its fellow of the opposite side, and with the musculophrenic and pericardiacophrenic arteries.
The lateral branch passes toward the side of the thorax, and anastomoses with the lower intercostal arteries, and with the musculophrenic. The lateral branch of the right phrenic gives off a few vessels to the inferior vena cava; and the left one, some branches to the esophagus.
Each vessel gives off superior suprarenal branches to the suprarenal gland of its own side. The spleen and the liver also receive a few twigs from the left and right vessels respectively.
The celiac artery is an essential source of blood, since the interconnections with the other major arteries of the gut are not sufficient to sustain adequate perfusion. Thus it cannot be safely ligated in a living person, and obstruction of the celiac artery will lead to necrosis of the structures it supplies.
They pass laterally and slightly upward, over the crur of the diaphragm, to the suprarenaL glands, where they anastomose with suprarenal branches of the inferior phrenic and renal arteries.
In the fetus these arteries are of large size.
Renal arteries
The renal arteries normally arise off the side of the abdominal aorta, immediately below the superior mesenteric artery, and supply the kidneys with blood. Each is directed across the crus of the diaphragm, so as to form nearly a right angle with the aorta.
The renal arteries carry a large portion of total blood flow to the kidneys. Up to a third of total cardiac output can pass through the renal arteries to be filtered by the kidneys.
The arterial supply of the kidneys is variable and there may be one or more renal arteries supplying each kidney. It is located above the renal vein.
Gonadal artery
The term gonadal artery is a generic term for a paired artery, with one arising from the abdominal aorta for each gonad. Specifically, it can refer to:
the testicular artery in males
the ovarian artery in females
lumbar arteries
The lumbar arteries are in series with the intercostals.
They are usually four in number on either side, and arise from the back of the aorta, opposite the bodies of the upper four lumbar vertebræ.
A fifth pair, small in size, is occasionally present: they arise from the middle sacral artery.
They run lateralward and backward on the bodies of the lumbar vertebræ, behind the sympathetic trunk, to the intervals between the adjacent transverse processes, and are then continued into the abdominal wall.
The arteries of the right side pass behind the inferior vena cava, and the upper two on each side run behind the corresponding crus of the diaphragm.
The arteries of both sides pass beneath the tendinous arches which give origin to the Psoa major, and are then continued behind this muscle and the lumbar plexus.
They now cross the Quadratus lumborum, the upper three arteries running behind, the last usually in front of the muscle.
At the lateral border of the Quadratus lumborum they pierce the posterior aponeurosis of the Transversus abdominis and are carried forward between this muscle and the Obliquus internus.
Inferior mesenteric artery
the inferior mesenteric artery, often abbreviated as IMA, supplies the large intestine from the left colic (or splenic) flexure to the upper part of the rectum, which includes the descending colon, the sigmoid colon, and part of the rectum. Proximally, its territory of distribution overlaps (forms a watershed) with the middle colic artery, and therefore the superior mesenteric artery. The SMA and IMA anastomose via the marginal artery (artery of Drummond). The territory of distribution of the IMA is more or less equivalent to the embryonic hindgut.
It descends in the middle line in front of the fourth and fifth lumbar vertebræ, the sacrum and coccyx, and ends in the glomus coccygeum (coccygeal gland).
From it, minute branches are said to pass to the posterior surface of the rectum.
On the last lumbar vertebra it anastomoses with the lumbar branch of the iliolumbar artery; in front of the sacrum it anastomoses with the lateral sacral arteries, and sends offsets into the anterior sacral foramina.
It is crossed by the left common iliac vein, and is accompanied by a pair of venæ comitantes; these unite to form a single vessel, which opens into the left common iliac vein.
The distribution of the common iliac artery is basically the pelvis and lower limb (as the femoral artery) on the corresponding side.
Both common iliac arteries are accompanied along their course by common iliac veins.